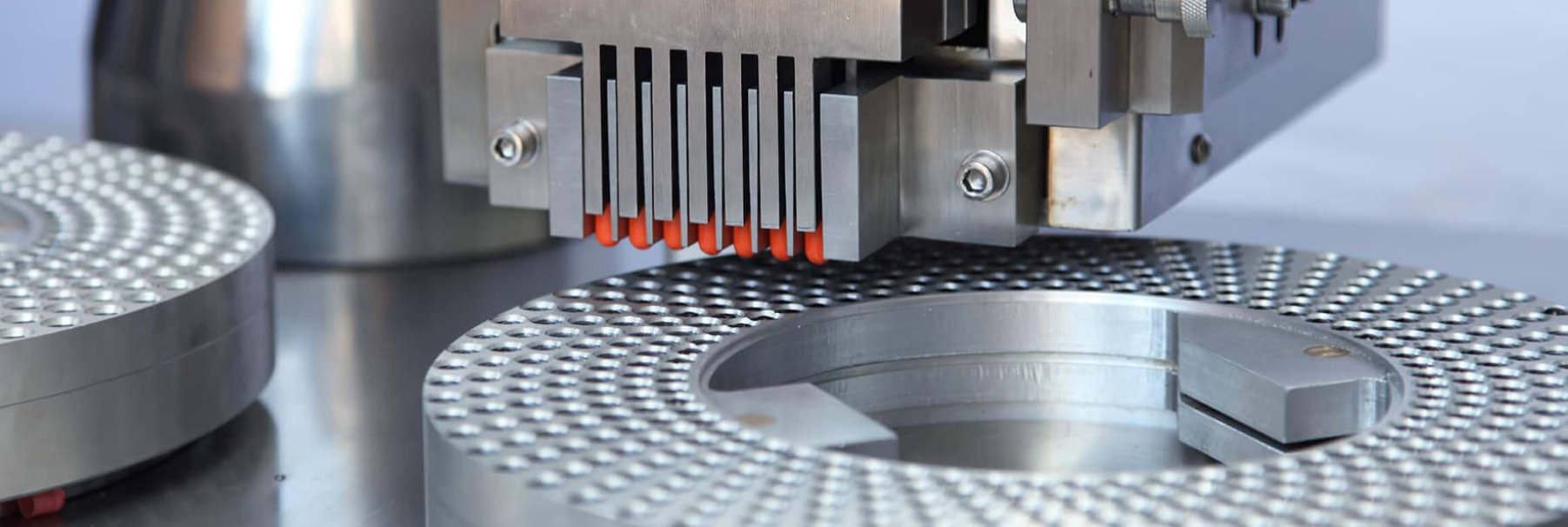
FORMULATION
Our talented team of formulators, scientists, chemists, and PhDs possess extensive experience in nutritional, biological, and pharmaceutical sciences and are driven to create products that meet your targeted criteria.
From single-ingredient oils to complex multi-ingredient fills, we have finished form solutions that appeal to many different kinds of consumers, including kids, adults, athletes, aging seniors, and everyone in between.
Starting with product discovery, we work with clients to develop new concepts to meet their specific needs. The formulation process then takes over, which works out the fine details of these innovations. By combining quality with services ranging from preliminary testing to delivery, our clients have everything they need to realize their products’ potential.